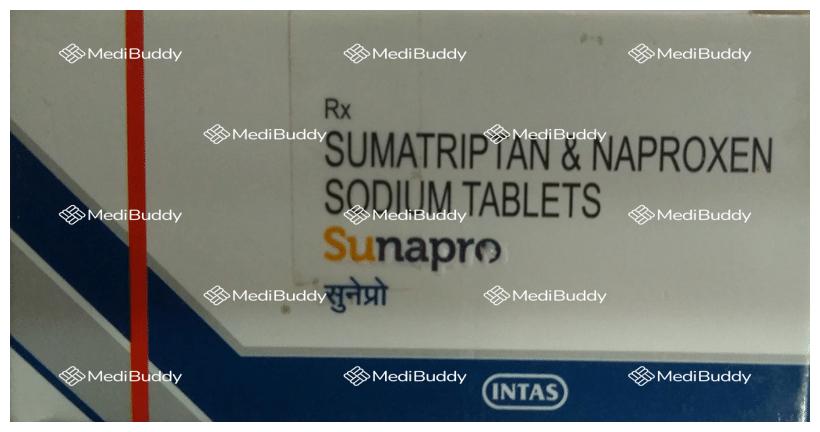
Sunapro Tablet
By Sunapro
Rx
2 Tablet in a Strip

Composition
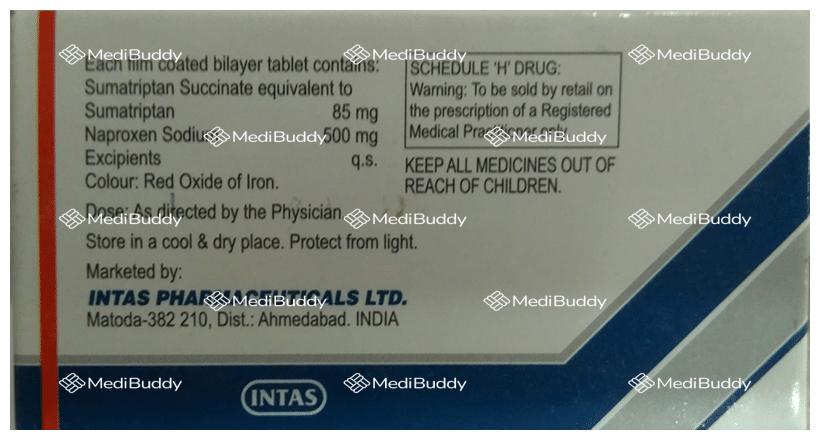
Sumatriptan(85mg) + Naproxen(500mg)

Manufacturer - Intas Pharmaceuticals Ltd
Chinubhai Centre, Off. Nehru Bridge, Ashram Road, Ahmedabad - 380009. Gujarat. India.

Expires on or after
October, 2025

liver
When taking Sunapro Tablet, it is important to be cautious if you have liver disease. Patients with liver conditions may require a dose adjustment when using this medication. It is advisable to seek guidance from your doctor before starting or continuing the use of Sunapro Tablet to ensure proper management and safety.

kidney
Sunapro Tablet needs caution in kidney disease patients. It may require dose adjustment. Consult your doctor for guidance.

alcohol
It is not safe to drink alcohol while taking Sunapro Tablet.

driving
Refrain from driving if you experience dizziness, depression, drowsiness, fatigue, or sleep disturbances while using Sunapro Tablet. These side effects may impair your ability to drive safely due to potential vision changes.

pregnancy
Sunapro Tablet is considered unsafe during pregnancy based on limited human studies. Animal studies suggest it may harm the developing baby. Your doctor will assess the benefits and risks before prescribing. Please consult your doctor.

breastfeeding
Sunapro Tablet is considered safe for breastfeeding moms. Studies show it has minimal transfer into breastmilk and is unlikely to harm the baby.
| Habit Forming | No |
| Chemical Class | - |
| Therapeutic Class | - |
| Action Class | - |
₹111.5
Inclusive of all taxes
Content verified by

Dr. Archana Prabhakar
MBBS, M.Med (Family Medicine)
Last update on 01-Oct-2024