Rifaxigyl-M Tablet
By Rifaxigyl-M
Rx
10 Tablet in a Strip

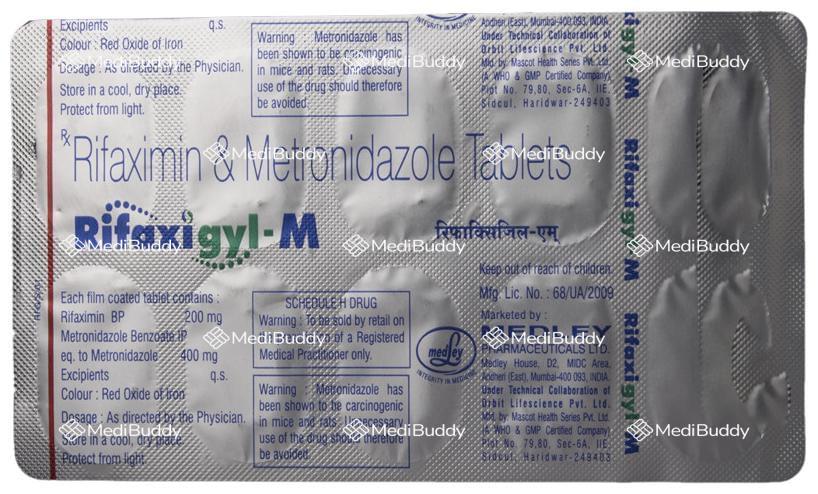
Composition
Rifaximin(200mg) + Metronidazole(400mg)

Manufacturer - Medley Pharmaceuticals
Medley House, D2, M.I.D.C. Area, Andheri (East), Mumbai-400 093, INDIA

Expires on or after
April, 2027

liver
When using Rifaxigyl-M Tablet, it is important to be cautious if you have liver disease. Patients with liver issues may require dose adjustments when taking this medication. It is recommended to consult with your doctor regarding the use of Rifaxigyl-M Tablet to ensure its safety and effectiveness for individuals with liver conditions.

kidney
Rifaxigyl-M Tablet appears safe for patients with kidney disease. Data shows dose adjustment may not be necessary. Consult your doctor for guidance.

alcohol
Caution is advised when consuming alcohol with Rifaxigyl-M Tablet, as it may result in symptoms like flushing, increased heart rate, nausea, thirst, chest pain, and low blood pressure (known as Disulfiram reaction).

driving
Caution - Driving Rifaxigyl-M Tablet may cause sleepiness, dizziness, confusion, hallucinations, fits, or temporary vision problems, which can impair driving ability.

pregnancy
Rifaxigyl-M Tablet may be unsafe during pregnancy, as animal studies suggest harm to the developing baby. Consult your doctor to evaluate benefits and risks before use.

breastfeeding
Rifaxigyl-M Tablet is likely safe to use while breastfeeding based on limited human data showing no significant risk to the baby.
| Habit Forming | No |
| Chemical Class | - |
| Therapeutic Class | GASTRO INTESTINAL |
| Action Class | - |
₹207
₹230
10% OFF
Inclusive of all taxes
Content verified by

Dr. Gowri Kulkarni
MBBS - General Medicine, DNB - Psychiatry, MRCGP [INT] Family Medicine, BSIC (BACP)
Last update on 11-Feb-2025