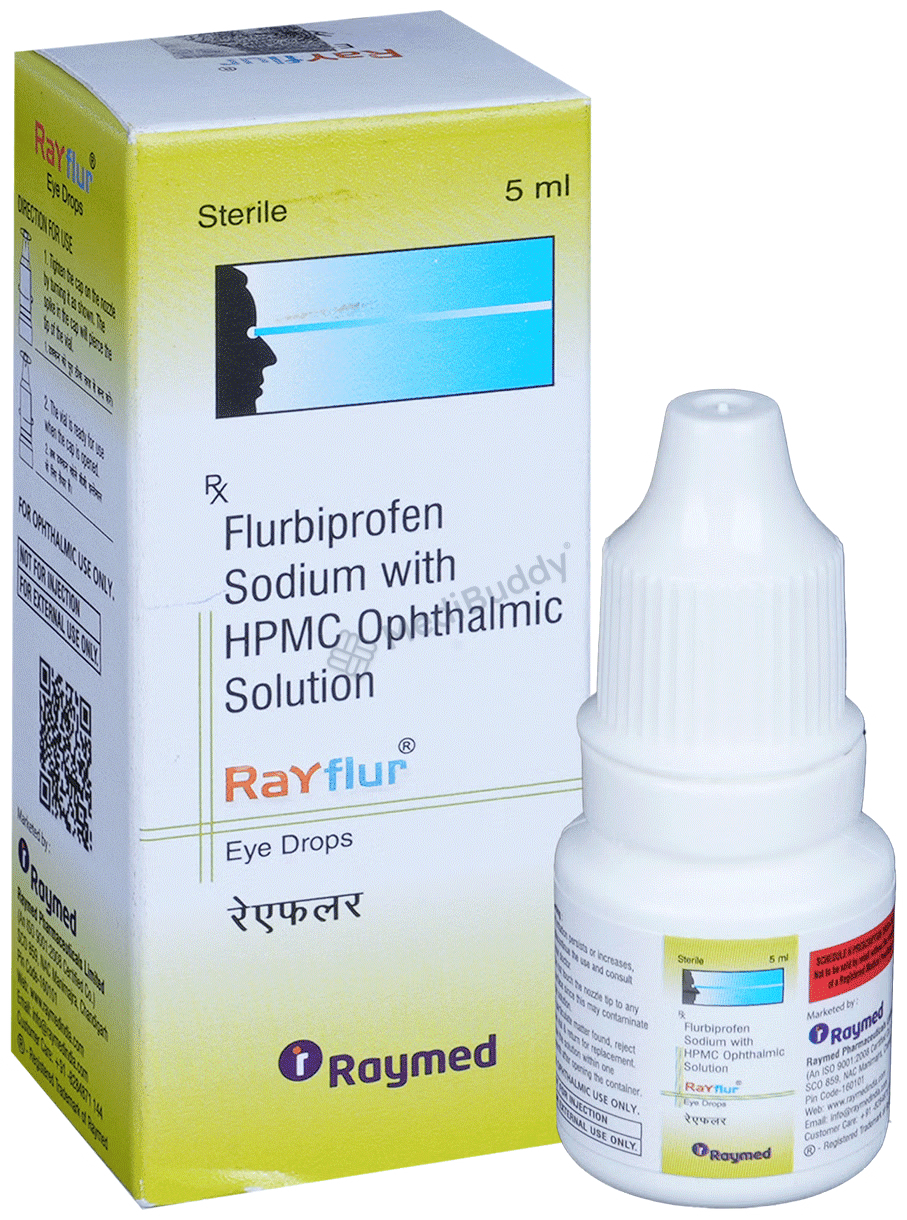
Rayflur Eye Drop
By Rayflur
Rx
5ml Eye Drop in a Packet

Composition
Flurbiprofen(NA) + Hydroxypropylmethylcellulose(NA) + Phenyl Mercuric Nitrate(NA)

Manufacturer - Raymed Pharmaceuticals Ltd
SCO 859, NAC Manimajra, Chandigarh-160101

Expires on or after
April, 2027

liver
For safety advice regarding Rayflur Eye Drop and liver issues, no interaction has been found or established according to the available data. It is recommended to consult with your doctor for proper guidance before using Rayflur Eye Drop.

kidney
No interaction or concerns related to kidney issues have been found or established with Rayflur Eye Drop.

alcohol
No interaction or known safety advice related to alcohol consumption with Rayflur Eye Drop.

driving
After using Rayflur Eye Drop, your vision may be blurry briefly. Avoid driving until your vision becomes clear.

pregnancy
Information on the safety of using Rayflur Eye Drop during pregnancy is not provided. It is best to seek advice from your doctor before using this product.

breastfeeding
It is better to consult your doctor before using Rayflur Eye Drop during breastfeeding.
| Habit Forming | No |
| Chemical Class | - |
| Therapeutic Class | OPHTHAL |
| Action Class | - |
₹71.5
Inclusive of all taxes
Content verified by

Dr. Abdullah Khan
MBBS - General Medicine
Last update on 11-Feb-2025