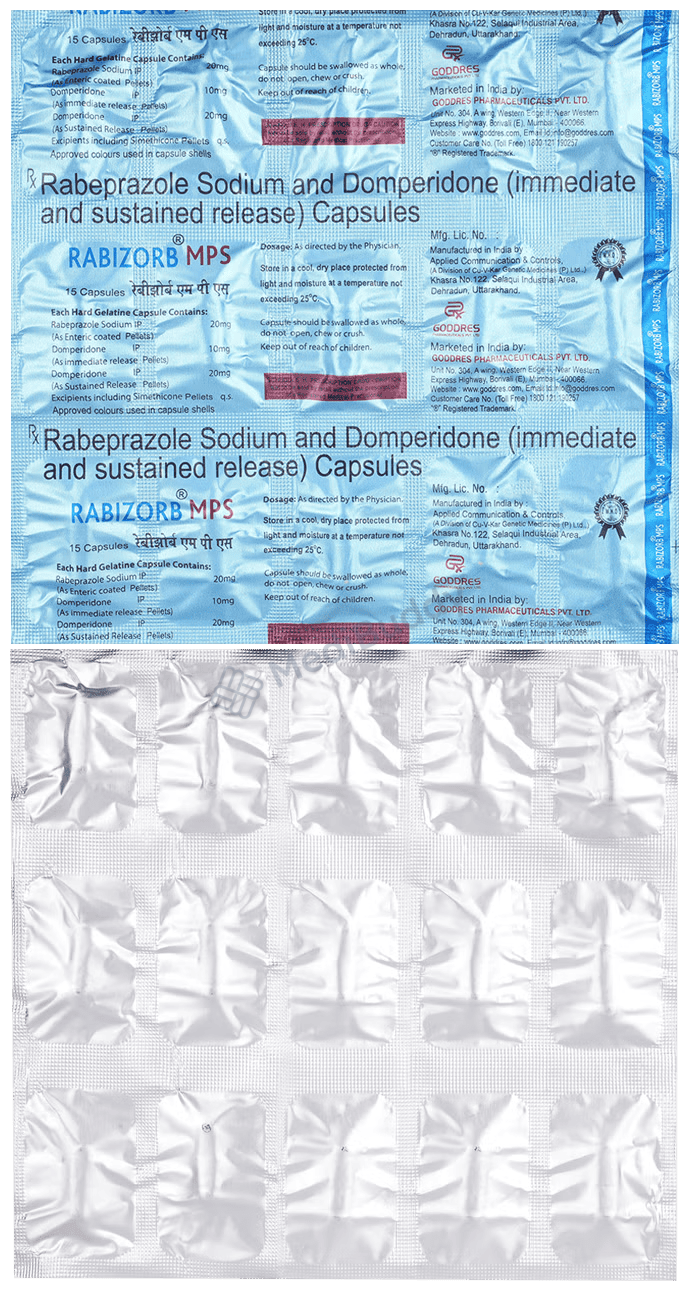
Rabizorb MPS Capsule SR
By Rabizorb MPS
Rx
15 Capsule IR in a Strip

Composition
Domperidone(30mg) + Rabeprazole(20mg)

Manufacturer - Goddres Pharmaceuticals Pvt Ltd
Unit No.304, A Wing, Western Edge II, Near Western Express Highway, Borivali (East), Mumbai – 400066.

Expires on or after
July, 2027

liver
When taking Rabizorb MPS Capsule SR, it is important to be cautious if you have liver disease. It may be necessary to adjust the dose of the capsule, so consulting with your doctor is essential. Patients with moderate and severe liver disease are advised not to use Rabizorb MPS Capsule SR Capsule to avoid any potential risks. Prior to use, it is crucial to discuss your liver condition with your healthcare provider to ensure safe and effective treatment.

kidney
Rabizorb MPS Capsule SR should be used carefully in patients with kidney disease. It may require dose adjustment, so consult your doctor.

alcohol
When taking Rabizorb MPS Capsule SR, it's important to be cautious if you consume alcohol. Consult with your doctor regarding alcohol intake.

driving
When taking Rabizorb MPS Capsule SR, be cautious as it can reduce alertness, impact vision, and cause drowsiness and dizziness. Avoid driving if experiencing these effects.

pregnancy
Rabizorb MPS Capsule SR may not be safe to use during pregnancy. Limited human studies suggest potential risks to the developing baby. Animal studies have shown harmful effects. Consult your doctor to weigh the benefits and risks.

breastfeeding
Rabizorb MPS Capsule SR could be unsafe during breastfeeding as limited data suggests it may pass into breastmilk and harm the baby.
| Habit Forming | No |
| Chemical Class | - |
| Therapeutic Class | GASTRO INTESTINAL |
| Action Class | - |
₹173.7
₹193
10% OFF
Inclusive of all taxes
Content verified by

Dr. Monie Riju Simon
MBBS - General Medicine
Last update on 01-Oct-2024