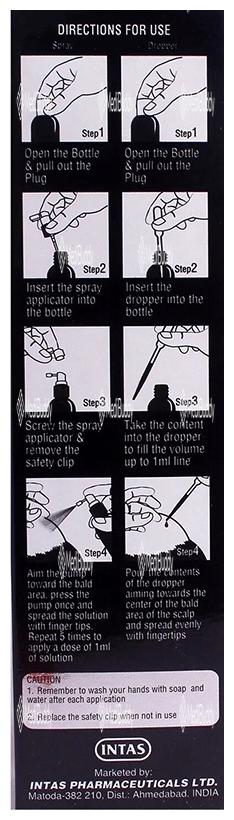
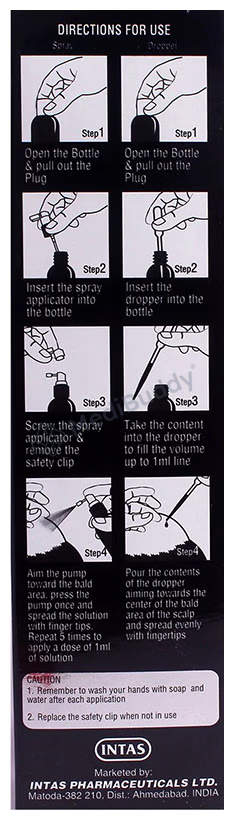
Morr 12.5% Solution
By Morr
60ml Solution in a Bottle

Composition
Minoxidil(12.5% w/v)

Manufacturer - Intas Pharmaceuticals Ltd
Chinubhai Centre, Off. Nehru Bridge, Ashram Road, Ahmedabad - 380009. Gujarat. India.

Expires on or after
June, 2026

liver
When considering the safety aspects of Morr 12.5% Solution in relation to the liver, it is important to note that no interactions have been found or established. However, caution is still advised when using this solution, especially if you have existing liver issues or concerns. It is recommended to consult with your doctor for proper guidance before using Morr 12.5% Solution to ensure the best course of action for your liver health.

kidney
No interaction has been found or established concerning Morr 12.5% Solution and kidney health. It is advisable to consult with your doctor for proper guidance before using the solution.

alcohol
Caution should be taken during pregnancy while using Morr 12.5% Solution with alcohol. No interactions have been found so far.

driving
Safety Advice for Driving while using Morr 12.5% Solution No interactions have been found related to driving. Nevertheless, always prioritize safety and be attentive while driving.

pregnancy
Morr 12.5% Solution is not recommended for use during pregnancy because animal studies have shown potential harm to the developing baby. Consult your doctor for a thorough assessment of the risks and benefits before considering its use.

breastfeeding
Morr 12.5% Solution is likely unsafe during breastfeeding. Limited data indicates potential harm to the baby through breastmilk. Not advised for women.
| Habit Forming | No |
| Chemical Class | Dialkylarylamines |
| Therapeutic Class | DERMA |
| Action Class | Potassium channel opener |
₹1290
Inclusive of all taxes
Content verified by

Dr. Archana Prabhakar
MBBS, M.Med (Family Medicine)
Last update on 01-Oct-2024