CTD-O 6.25/20 Tablet
By CTD-O
Rx
10 Tablet in a Strip

Composition
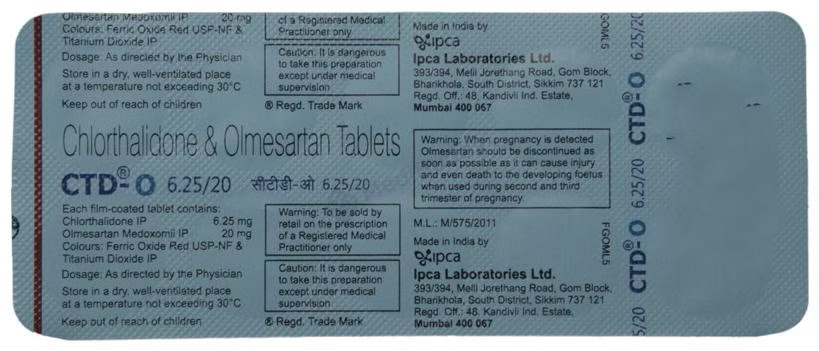
Olmesartan Medoxomil(20mg) + Chlorthalidone(6.25mg)

Manufacturer - Ipca Laboratories Ltd
142 AB, Kandivli Industrial Estate, Kandivli (West), Mumbai - 400 067, Maharashtra

Expires on or after
October, 2026

liver
When using CTD-O 6.25/20 Tablet, it's important to be cautious if you have a liver disease. You may need a dose adjustment, so always consult your doctor before taking this medication. It's advisable not to use CTD-O 6.25/20 Tablet if you have severe liver disease. Remember to seek medical advice to ensure the safe use of this medication.

kidney
CTD-O 6.25/20 Tablet should be used carefully by patients with severe kidney disease. Dosage adjustment might be necessary, so consulting your doctor is advised. Avoid using CTD-O 6.25/20 Tablet in these cases.

alcohol
CTD-O 6.25/20 Tablet can increase drowsiness when taken with alcohol.

driving
CTD-O 6.25/20 Tablet may cause reduced alertness, blurred vision, drowsiness, and dizziness. Avoid driving if experiencing these effects.

pregnancy
CTD-O 6.25/20 Tablet is not safe to use during pregnancy due to potential risks to the developing baby. However, in life-threatening situations, a doctor may prescribe it if benefits outweigh risks. Always consult your doctor.

breastfeeding
CTD-O 6.25/20 Tablet should be used with caution during breastfeeding, as it is considered unsafe. Limited human data indicates that the drug may pass into breastmilk and potentially harm the baby.
| Habit Forming | No |
| Chemical Class | - |
| Therapeutic Class | CARDIAC |
| Action Class | - |
₹154.25
Inclusive of all taxes
Content verified by

Dr. Abdullah Khan
MBBS - General Medicine
Last update on 01-Oct-2024